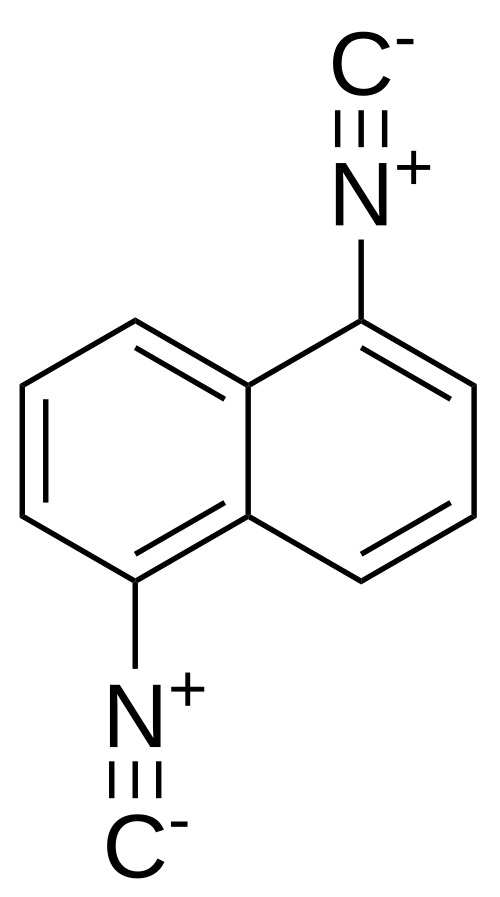
1,5-Diisocyanonaphthalene
 | |
| Names | |
|---|---|
| IUPAC name
Naphthalene-1,5-diyldiisocyanide
| |
| Other names
1,5-Diisocyanonaphthalene; DIN; 1,5-naphthalenediyldiisocyanide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C12H6N2 | |
| Molar mass | 178.194 g·mol−1 |
| Appearance | Solid |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Laboratory chemical; handle with standard precautions |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
1,5-Diisocyanonaphthalene (DIN) is an aromatic diisocyanide (isonitrile) in which two –N≡C groups occupy the 1- and 5-positions of the naphthalene ring. The compound is also named 1,5-naphthalenediyldiisocyanide and has the molecular formula C12H6N2 and relative molar mass 178.19 g·mol−1.[1] It has been studied as a photophysical probe and as a lead compound for antifungal research.
Structure and properties
Isocyanides feature the –N≡C functional group (often depicted as –N+≡C− ↔ –N=C:), bonded to the aromatic ring through nitrogen. DIN's two isocyanide substituents make it a rigid, weakly polar π-system. Photophysically, DIN shows limited solvatochromism compared with mono-formamido/mono-isocyano analogues (e.g., ICNF), but it forms notable π-complexes with aromatic solvents that modulate emission, enabling discrimination among aromatics by fluorescence.[2]
Synthesis
1,5-Diisocyanonaphthalene can be obtained by converting 1,5-diaminonaphthalene to the corresponding N,N′-diformamide, followed by dehydration (e.g., with phosphorus oxychloride in the presence of base). Reaction conditions and solvent choice influence outcome for this system.[3]
Applications
Fluorescence/analytical: DIN's emission responds to specific π–π interactions in aromatic solvents, suggesting use in distinguishing aromatic hydrocarbons optically; related hydrolysis-derived ICNF is a strongly solvatochromic dye.[2]
Antifungal activity
In standardized CLSI broth microdilution tests against three Aspergillus fumigatus strains (MYA 3627, ATCC 204305 and Af293), 1,5-diisocyanonaphthalene showed the lowest MIC values among the tested isocyano-naphthalene derivatives, with **MIC = 0.6 µg·mL−1 (3.4 µM)** for all strains. Practical use may be limited by poor aqueous solubility; a related dimethylamino analogue (DIMICAN) progressed to in-vivo evaluation in a murine aspergillosis model.[4]
See also
- Isocyanide
- Naphthalene
References
- ^ "1,5-Naphthalenediyldiisocyanide". pubchem.ncbi.nlm.nih.gov. Retrieved 19 August 2025.
- ^ a b Kopcsik, Erika; Mucsi, Zoltán; Schiwert, Rajmond; Vanyorek, László; Viskolcz, Béla; Nagy, Miklós (2025-01-03). "Aromatic pi-complexation of 1,5-diisocyanonaphthalene with benzene derivatives". Scientific Reports. 15 (1) 629. Bibcode:2025NatSR..15..629K. doi:10.1038/s41598-024-84769-3. PMC 11698713. PMID 39753877.
- ^ Kopcsik, Erika; Mucsi, Zoltán; Kontra, Bence; Vanyorek, László; Váradi, Csaba; Viskolcz, Béla; Nagy, Miklós (2023). "Preparation and Optical Study of 1-Formamido-5-Isocyanonaphthalene, the Hydrolysis Product of the Potent Antifungal 1,5-Diisocyanonaphthalene". International Journal of Molecular Sciences. 24 (9): 7780. doi:10.3390/ijms24097780. PMC 10177923. PMID 37175485.
- ^ Szigeti, Zsuzsa Máthéné; Tálas, László; Széles, Adrienn; Hargitai, Zoltán; Nagy, Zsolt László; Nagy, Miklós; Kiss, Alexandra; Kéki, Sándor; Szemán-Nagy, Gábor (2022). "Potential Original Drug for Aspergillosis: In Vitro and In Vivo Effects of 1-N,N-Dimethylamino-5-Isocyanonaphthalene (DIMICAN) on Aspergillus fumigatus". Journal of Fungi. 8 (10): 985. doi:10.3390/jof8100985. PMC 9605569. PMID 36294550.