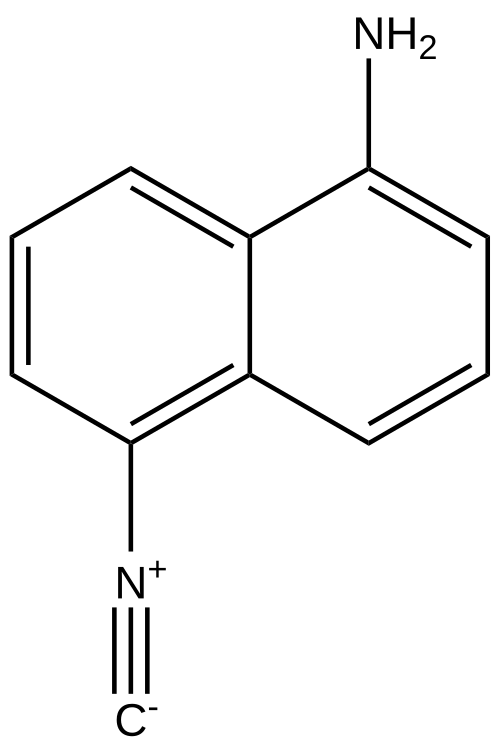
1-Isocyano-5-aminonaphthalene
 | |
| Names | |
|---|---|
| IUPAC name
1-Isocyano-5-aminonaphthalene
| |
| Other names
5-Amino-1-isocyanonaphthalene; ICAN
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C11H8N2 | |
| Molar mass | 168.199 g·mol−1 |
| Appearance | Yellow–orange solid (reported) |
| Low in water; soluble in organic solvents | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
1-Isocyano-5-aminonaphthalene (commonly ICAN) is a substituted naphthalene bearing an isocyano (–N≡C) group and an amino (–NH2) group in a 1,5-relationship. The push–pull pairing of an electron donor (amino) and an electron-withdrawing (isocyano) group gives ICAN a pronounced intramolecular charge-transfer (ICT) character, which underlies its strong solvatochromic fluorescence and its use as an environment-sensitive fluorophore.[1][2] ICAN and several N-alkylated analogues have also been explored as antifungal leads, with reports of low minimum inhibitory concentrations (MICs) against Candida spp. and proof-of-concept efficacy of a dimethylated derivative in a neutropenic mouse model.[3]
Structure and photophysics
The 1,5-arrangement of –NH2 and –N≡C on the naphthalene core creates a donor–acceptor system. ICAN exhibits large Stokes shifts and marked solvent-dependent absorption and emission changes; related ICAN isomers (e.g., 1,4-ICAN; 2,6-ICAN) show systematic variations consistent with ICT tuning by the substitution pattern.[1][2]
Synthesis
ICAN is prepared by the carbylamine (Hofmann) reaction, in which a primary amine is converted to an isocyanide via in situ dichlorocarbene generated from chloroform and base (often with phase-transfer catalysis). For naphthalene systems, treating the appropriate aromatic diamine under carbylamine conditions affords the mono-isocyanide (ICAN) together with minor amounts of the diisocyanide.[4][1]
By contrast, the related 1,5-diisocyanonaphthalene (DIN) is efficiently obtained by formylation of both amines to a diformamide followed by POCl3-based dehydration; under controlled acidic conditions DIN undergoes partial hydrolysis to give the nonsymmetric 1-formamido-5-isocyanonaphthalene (ICNF).[5][6]
Properties and uses
Fluorescence and solvatochromism
ICAN's emission shifts from blue–green in less polar media toward orange–red in polar/protic media; it has been applied as an environment-sensitive probe and as a reference push–pull dye in photophysics.[1][2]
Chemical sensing
ICAN-type dyes have been used in complexation/ion-sensing studies (e.g., silver(I) detection) and for background reduction in biolabelling.[7]
Antifungal research
ICAN, its N-alkyl derivatives, and DIN show in vitro activity against Candida spp.; the dimethylated analogue DIMICAN achieved MIC values as low as 0.04–1.25 μg·mL−1 against clinical isolates and improved survival in a neutropenic mouse model of invasive candidiasis (5 mg·kg
1 i.p.).[3]
Safety
ICAN and related solids are often described as having little or no noticeable odour (likely reflecting low volatility); ICAN and its derivatives are reported as odorless . In general, many low-molecular isocyanides are strongly malodorous, although exceptions exist.[8]
Derivatives
| Abbrev. | Substituents (1;5) | Photophysical notes (qual.) | Antifungal notes |
|---|---|---|---|
| ICAN | –N≡C; –NH2 | Strong ICT; pronounced solvatochromism | Active vs Candida spp. (µg·mL1 range).[3] |
| MICAN | –N≡C; –NHCH3 | Emission shifted vs ICAN | Active; improved MICs vs ICAN.[3] |
| DIMICAN | –N≡C; –N(CH3)2 | Red-shifted emission; strong ICT | Most potent in set; MIC 0.04–1.25 µg·mL1; in-vivo efficacy in mice.[3] |
| DIN | –N≡C; –N≡C | Rigid, lower aqueous solubility | Low MICs in vitro; solubility limits addressed by formulation.[3] |
References
- ^ a b c d Rácz, D.; Nagy, M.; Mándi, A.; Zsuga, M.; Kéki, S. (2013). "Solvatochromic properties of a new isocyanonaphthalene based fluorophore". Journal of Photochemistry and Photobiology A: Chemistry. 270: 19–27. Bibcode:2013JPPA..270...19R. doi:10.1016/j.jphotochem.2013.07.007.
- ^ a b c Kovács, S. L.; Nagy, M.; Fehér, P. P.; Zsuga, M.; Kéki, S. (2019). "Effect of the Substitution Position on the Electronic and Solvatochromic Properties of Isocyanoaminonaphthalene (ICAN) Fluorophores". Molecules. 24 (13): 2434. doi:10.3390/molecules24132434. PMC 6678642. PMID 31284476.
- ^ a b c d e f Nagy, M.; Szemán-Nagy, G.; Kiss, A.; Nagy, Z. L.; Tálas, L.; Rácz, D.; Majoros, L.; Tóth, Z.; Szigeti, Z. M.; Pócsi, I.; et al. (2020). "Antifungal Activity of an Original Amino-Isocyanonaphthalene (ICAN) Compound Family: Promising Broad Spectrum Antifungals". Molecules. 25 (4): 903. doi:10.3390/molecules25040903. PMC 7070524. PMID 32085460.
- ^ Gokel, G. W.; Widera, R. P.; Weber, W. P. (1976). "Phase-Transfer Hofmann Carbylamine Reaction: tert-Butyl Isocyanide". Organic Syntheses. 55: 96–99. doi:10.15227/ORGSYN.055.0096.
- ^ Salami, S. A.; Siwe-Noundou, X.; Krause, R. W. M. (2022). "A More Sustainable Isocyanide Synthesis from N-Substituted Formamides Using Phosphorus Oxychloride in the Presence of Triethylamine as Solvent". Molecules. 27 (20): 6850. doi:10.3390/molecules27206850. PMC 9608669. PMID 36296444.
- ^ Kopcsik, E.; Nagy, M.; Kéki, S. (2023). "Preparation and Optical Study of 1-Formamido-5-Isocyanonaphthalene, the Hydrolysis Product of the Potent Antifungal 1,5-Diisocyanonaphthalene". International Journal of Molecular Sciences. 24 (9): 7780. doi:10.3390/ijms24097780. PMC 10177923. PMID 37175485.
- ^ Nagy, M.; Rácz, D.; Nagy, Z. L.; Fehér, P. P.; Kalmár, J.; Fábián, I.; Kiss, A.; Zsuga, M.; Kéki, S. (2018). "Solvatochromic isocyanonaphthalene dyes as ligands for silver(I) complexes, their applicability in silver(I) detection and background reduction in biolabelling". Sensors and Actuators B: Chemical. 255: 2555–2567. Bibcode:2018SeAcB.255.2555N. doi:10.1016/j.snb.2017.09.061.
- ^ Massarotti, Alberto; Brunelli, Francesca; Aprile, Silvio; Giustiniano, Mariateresa; Tron, Gian Cesare (2021). "Medicinal Chemistry of Isocyanides". Chemical Reviews. 121 (10): 7251–7341. doi:10.1021/acs.chemrev.1c00143. PMID 34197077.